中国科学院微生物研究所,中国微生物学会,中国菌物学会
文章信息
- Liangting Liu, Xiang Xiao, Yu Zhang. 2021
- 刘亮霆, 肖湘, 张宇. 2021
- Environmental adaptation basis and ecological function of deep-sea ammonia-oxidation archaea
- 深海氨氧化古菌的环境适应基础及生态功能
- Acta Microbiologica Sinica, 61(9): 2643-2662
- 微生物学报, 61(9): 2643-2662
-
文章历史
- 收稿日期:2020-12-15
- 修回日期:2021-02-24
- 网络出版日期:2021-06-16
2. State Key Laboratory of Ocean Engineering, Shanghai Jiao Tong University, Shanghai 200240, China;
3. School of Oceanography, Shanghai Jiao Tong University, Shanghai 200240, China;
4. International Center for Deep Life Investigation(IC-DLI), Shanghai Jiao Tong University, Shanghai 200240, China;
5. Laboratory for Marine Biology and Biotechnology, Pilot National Laboratory for Marine Science and Technology, Qingdao 266237, Shandong Province, China
2. 上海交通大学海洋工程国家重点实验室, 上海 200240;
3. 上海交通大学海洋学院, 上海 200240;
4. 上海交通大学深部生命国际研究中心, 上海 200240;
5. 青岛海洋科学与技术试点国家实验室, 海洋生物学与生物技术功能实验室, 山东 青岛 266237
The discovery of ammonia-oxidizing archaea (AOA) is an important milestone[1] that changed the understanding of archaea and the nitrogen cycle[2–4]. AOA uses energy produced by aerobic ammonia oxidation to synthesize organic matter, and its total stoichiometric amount of ammonia oxidation is indistinguishable from ammonia-oxidizing bacteria (AOB)[5]. Based on phylogenetics analysis, it is likely that AOA originated during the Great Oxygenation Event around 2.3 billion years ago. They evolved from anaerobic terrestrial non-AOA Thaumarchaeota, and their habitats have steadily expanded from land to the shallow and deep sea[6]. Lateral gene transfer (LGT) from bacteria to archaea has occurred continuously throughout the evolutionary history of AOA from land to deep sea[6], which may be one reason for their high diversity. The latest edition of the Bergey's Manual of Systematics of Archaea and Bacteria classifies AOA into four orders and nine genera[7]. AOA assigned to 18 branches of the genus level in addition to four main branches of the order level [NP (Nitrosopumilales), NT (Ca. Nitrosotaleales), NS (Nitrososphaerales), and NC (Ca. Nitrosocaldales)][8], based on the amino monooxygenase subunit A (amoA) gene sequences of archaea. At present, AOA have been isolated or enriched from natural and artificial ecosystems such as shallow seawater[9–11], shallow marine sediments[9, 12], terrestrial hot springs[13–16], frozen soil[17], garden soil[18], agricultural soil[19–24], aquariums[1, 25], and sewage treatment plants[26–28]. However, the cultured AOA mostly belong to a few evolutionary branches at the genus level, such as Nitrososphaera (NS-α), Ca. Nitrosocosmicus (NS-ζ), Ca. Nitrosocaldus (NC-α), Ca. Nitrosoarchaeum (NP-γ), Nitrosopumilus (NP-γ), Ca. Nitrosotenuis (NP-η), Ca. Nitrosopelagicus (NP-ε) and Ca. Nitrosotalea (NT-α) and these cultured groups are not the most abundant in the environment[8]. Many uncultured groups have high abundance in the environment, such as NS-γ, NS-δ, NP-α[8]. The ecological functions of these uncultured AOA in the environment are worth to be explored.
AOA have achieved great ecological success. Apart from ubiquitous on Earth, AOA also occupy a high number of cells in various environments. AOA account for 1%–5% of all prokaryotes in terrestrial ecosystems[29–30]. Their abundance is much higher than AOB in acidic or alkaline soils[31–32]. About 1×1028 AOA cells in the ocean account for 20%–40% of all prokaryotes in the seawater[33–34], as a dominant microbial taxon in deep seawater. The distribution of AOA in the ocean has a spatial distribution specificity. For example, In Challenger Deep of the Mariana Trench, the abundance of AOA is extremely low in seawater at depths less than 100 m. However, they are the most abundant microorganisms in the mesopelagic (200–1000 m), bathyal (1000–4000 m), and abyssal (4000–6000 m) zone, accounting for up to 45%–80% of the total prokaryotes (inferring to 16S rRNA gene abundances, similarly hereinafter). Even in the hadal zone (6000–11000 m), the relative abundance of AOA in prokaryotes can still reach 10%–30%[34]. At depths deeper than 200 m, AOA nearly occupy all archaeal biomass, accounting for as much as 96.0%–99.6% of the relative abundance of archaeal 16S rRNA gene abundance[34]. The cell number of prokaryotes in seawater decreases with increasing depth, but the cell number of AOA shows a trend of rising then falling, with the highest one in the 100–1000 m depth range[33–34]. In surface sediments, AOA accounts for up to 30% of the prokaryotes, but its abundance decreases from the seafloor down[35].
Marine AOA belong to the order Nitrosopumilales (Group I.1a, MG-I, NP). Based on the amoA gene sequence, Nitrosopumilales is divided into eight branches as NP-α, β, γ, δ, ε, ζ, η, θ at the genus level[8], and their distribution is dependent on the depth of the seawater (Figure 1). For instance, NP-α (dominant in the mesopelagic, bathyal, abyssal and hadal zone), NP-γ (lower abundance in all depths and relatively higher abundance in the hadal zone), NP-δ (present in surface waters, low abundance), and NP-ε (dominant in surface water)[8, 36]. At present, most of the cultured marine AOA belong to NP-γ, although they inhabit seawater at depths of 50–10000 m, their relative abundance is very low (Figure 1). There is a lack of cultures that are dominant in the deep sea (Figure 1, Table 1), and their metabolic characteristics at the cellular level and the physiological basis for adaptation to the extreme environment of the deep sea have not been confirmed under laboratory conditions.
| AOA strains | Lineagea | Year | Source (depth/m) | Relative abundance/% | Enrichment duration |
| Nitrosopumilus maritimus SCM1 | NP-γ | 2005 | Marine aquarium | 100 | N/A |
| Ca. Nitrosopumilus koreensis AR1 | NP-γ | 2010 | Marine sediment (78) | > 80 | 2 years |
| Nitrosopumilus ureiphilus PS0 | NP-γ | 2014 | Marine sediment (50) | 100 | N/A |
| Nitrosopumilus cobalaminigenes HCA1 | NP-γ | 2014 | Seawater (50) | 100 | N/A |
| Ca. Nitrosopelagicus brevis CN25 | NP-ε | 2015 | Seawater (25) | 90-95 | N/A |
| Nitrosopumilus maritimus NAO2 | NP-γ | 2015 | Seawater (5) | 100 | N/A |
| Nitrosopumilus maritimus NAO6 | NP-γ | 2015 | Seawater (5) | 100 | N/A |
| Ca. Nitrosopumilus piranensis D3C | NP-γ | 2016 | Seawater (0.5) | > 99 | 2 years |
| Ca. Nitrosopumilus adriaticus NF5 | NP-γ | 2016 | Seawater (0.5) | > 99 | 2 years |
| Nitrosopumilus sp. DDS1 | NP-γ | 2016 | Seawater (200) | 100 | 2 years |
| Nitrosopumilus oxyclinae HCE1 | NP-γ | 2017 | Seawater (17) | 100 | N/A |
| Ca. Nitrosopumilus sp. NM25 | NP-γ | 2011 | Coastal sand | 89 | 2 years |
| Ca. Nitrososphaera gargensis Ga9.2 | NS-α | 2008 | Hot spring | 50 | 6 years |
| Ca. Nitrosocaldus yellowstonii HL72 | NC-α | 2008 | Hot spring | > 90 | 2 years |
| Ca. Nitrosotenuis uzonensis N4 | NP-η | 2013 | Hot spring | 50 | 7 years |
| Ca. Nitrosocaldus islandicus 3F | NC-α | 2018 | Hot spring biofilm | 85 | N/A |
| Ca. Nitrosocaldus cavascurensis SCU2 | NC-α | 2018 | Hot spring mud | 92 | 4 years |
| Ca. Nitrosotenuis cloacae SAT1 | NP-η | 2016 | Wastewater treatment plant | 91 | 1 year |
| Ca. Nitrosocosmicus exaquare G61 | NS-ζ | 2017 | Wastewater treatment plant | 99 | 3 years |
| Ca. Nitrososphaera sp. OTU8 | NS-α | 2017 | Wastewater treatment plant | 91 | N/A |
| Ca. Nitrosotenuis aquarius AQ6F | NP-η | 2018 | Freshwater aquarium biofilter | 97-99 | N/A |
| Ca. Nitrosoarchaeum limnia SFB1 | NP-γ | 2011 | Estuarine sediment | 84 | N/A |
| Ca. Nitrosocosmicus oleophilus MY3 | NS-ζ | 2016 | Coal tar-contaminated sediment | > 99 | N/A |
| Ca. Nitrosocosmicus arcticus Kfb | NS-ζ | 2019 | Frozen soil | 72-93 | 5 years |
| Nitrososphaera viennensis EN76 | NS-α | 2011 | Garden soil | 100 | 2 years |
| Ca. Nitrosotalea devanaterra Nd1 | NT-α | 2011 | Acid soil | 90 | N/A |
| Ca. Nitrosotalea sp. Nd2 | NT-α | 2014 | Acid agricultural soil | 100 | 3 years |
| Ca. Nitrosoarchaeum koreensis MY1 | NP-γ | 2011 | Agricultural soil | 90 | 2 years |
| Ca. Nitrososphaera sp. JG1 | NS-α | 2012 | Agricultural soil | 89 | 1 year |
| Ca. Nitrososphaera evergladensis SR1 | NS-α | 2014 | Agricultural soil | 50 | 1 year |
| Ca. Nitrosotenuis chungbukensis MY2 | NP-η | 2014 | Agricultural soil | 91 | 3 years |
| Ca. Nitrosocosmicus franklandus C13 | NS-ζ | 2016 | Agricultural soil | 100 | N/A |
| Ca. Nitrosocosmicus agrestis SS | NS-ζ | 2019 | Agricultural soil | > 97 | 150 days |
| Lineage a: compatible with taxonomy based on archaeal amoA[8]. | |||||
1 Habitat of deep-sea ammonia- oxidizing archaea 1.1 Space
The ocean covers 71% of the Earth's surface area (3.62×108 km2) and contains 97% of the total water volume (1.33×109 km3), with an average depth of 3800 m and a depth approaching 11000 m in the Mariana Trench. The sea at a depth of 1000 m or more is called the deep sea, with an area of 88% (3.18×108 km2) and a volume of 75% (1.143×109 km3) of the total ocean[37], occupying the most extensive area of the biosphere. Despite the small area depth of the hadal zone at depths greater than 6000 m, which is equivalent to China's land area, it covers 45% of the ocean depth.
1.2 Hydrostatic pressureHydrostatic pressure is one of the distinguishing features of the ocean, and its magnitude is linked to the depth of seawater, and the hydrostatic pressure in the deep sea is up to 1.15×108 Pa. Pressure induces a phase change from liquid to solid. It affects Gibbs free energy, conducive to the chemical reaction accompanying the decrease in volume (Le Chatelier's principle). However, the pressure-induced volume change is generally small in non-gaseous reactions[38]. Most covalent bonds involved in the primary structure of proteins are pressure stable at least at (1.0–1.5)×108 Pa[38]. Therefore, the pressure mainly affects the intermolecular forces of proteins, such as stabilizing hydrogen bonds, reducing electrostatic interactions, and breaking down hydrophobic interactions, thereby affecting protein hydration, folding, unfolding and aggregation of proteins, and even causing denaturation[39]. Under high hydrostatic pressure (HHP), the volume of liquid decreases, and the volume change is much greater for hydrocarbons relative to water[40]. Under the effects of HHP, the phospholipid bilayer is compressed, and the acyl chains are tightly packed, resulting in a phase change to gelatinous liquid crystals[41], leading to a decrease in fluidity. Increasing the pressure at different temperatures can denature or renature DNA[42], but under environmental parameters of deep sea, HHP acts to stabilize the double helix structure of DNA.
1.3 Low temperaturesExcept for the space around hydrothermal vents, the deep-sea temperature is only 2–3 ℃. In addition to pressure, kinetics of biochemical enzymatic reaction and phase transitions of biomacromolecules are closely related to temperature. Low temperature leads to a significant decrease in the rate of enzymatic reactions and membrane fluidity[43]. Like high pressure, low temperatures also affect the hydrophobic interactions and hydrogen bonds between protein tertiary structures, enhancing hydration and even leading to cold denaturation. Besides, increasing the solubility of oxygen at low temperatures may be traced to an increase in reactive oxygen species (ROS)[44].
1.4 BiogeochemistryMarine photosynthesis occurs at a water depth of 0–200 m. As the depth increases, the sunlight gradually decreases until it completely disappears. There is a faint blue light in the range of 200–1000 m, while the space deeper than 1000 m has no sunlight at all. Usually, the deep sea is an oligotrophic environment, where the available organic matter is mainly derived from the ocean surface[45–46]. Nevertheless, only a small fraction of this generally settle to the deep sea. A study in Iquique, northern Chile, found that 82% of the proteins produced by photosynthesis were degraded at 0–30 m depth, another 15% degraded at 30–300 m, and only about 1% reached the surface sediments at 1200 m depth[47]. Many studies have shown that large AOA produce additional organic matter to the deep-sea environment by fixing inorganic carbon, about 400 million tons of carbon annually[48–49]. The equilibrium of CO2, HCO3– and CO32– is pH-dependent, and inorganic carbon is predominantly in the form of HCO3– in seawater. In the deep sea, ammonia is a major electron donor. In most oceans, the primary source of ammonia is the euphotic layer or land[50]. Inorganic or organic nitrogen input from terrestrial runoff and ammonia synthesized by nitrogen-fixing microorganisms is utilized by plankton to synthesize biomass. These nitrogen-containing biomasses are decomposed after the death of the organisms, with some of them settling in the deep sea in the form of particulate organic matter (POM)[51–53]. The POMs rely on the high temperature of hydrothermal fluids or the mineralization by bacteria with extracellular enzymatic activity to release ammonia[54]. Recent studies have demonstrated that nitrogen fixation by methane anaerobic oxidation-sulfate reducing microorganisms may also be an essential source of ammonia in the deep sea[55]. In contrast to ammonia limitation, nitrate concentration in the deep sea is significantly higher under nitrification[56]. Influenced by low temperatures and high pressure, the oxygen concentration in deep seawater is lower than that of the surface layer of the ocean, but higher than the oxygen minimum zone (OMZ)[57] (Figure 1).
2 Deep sea is a shelter for AOAThe low cell number and extremely low relative abundance in the surface indicate that AOA does not possess competing advantage in the surface seawater. From the perspective of environmental factors, surface seawater temperature, pH, dissolved oxygen concentration, and light intensity are significantly higher than those in deep-sea seawater. AOA adapt to a wide temperature range, and the optimal growth temperature is generally greater than 20 ℃. Therefore, the temperature of surface seawater does not limit the inhabitant of AOA.
The pH of seawater is alkaline, generally between 7.5 and 8.4. Surface seawater has the highest pH due to the influence of phytoplankton photosynthesis; as the depth increases, the seawater pH gradually decreases. The alkali tolerance of AOA is relatively low compared to AOB. It has been shown that the viable pH for marine AOA growth is in the range of 5.9–8.1, and the optimal pH is generally between 6.8–7.3[58]. The physiological characteristics of the isolated strains indicate that the high pH of shallow seawater likely inhibits AOA growth. In contrast, the deep sea provides a relatively low pH environment conducive to AOA growth.
Although AOA are aerobic microorganisms, currently isolated shallow-sea AOA are sensitive to ROS[59]. Because they do not have a complete antioxidant system (encoding superoxide dismutase, but lack of catalase, peroxidase). Ammonia oxidation of marine AOA are inhibited by 10 nmol/L H2O2[60], which may be related to the origin of AOA from anaerobic microorganisms[6]. The dissolved oxygen on the sea surface is close to saturation. Although dissolved oxygen concentration decreases rapidly with the increasing depth, it is still much higher than the deep sea in the range of 0–100 m deep, resulting in ROS stress on shallow-sea AOA
Illumination is one of the most different environmental factors between the deep and shallow oceans. Only about 1% of light energy can pass through 100 m of seawater. High light intensity markedly inhibits the activity of ammonia-oxidizing microorganisms, regardless of AOA and AOB. The mechanism of photoinhibition of ammonia-oxidizing microorganisms is that photooxidation destroys ammonia monooxygenase (AMO). Light induces a reaction between oxygen and organic matter[61], increasing the ROS concentration of light environment by 1–2 orders of magnitude. The reaction between oxygen and organic matter is photocatalyzed, increasing ROS concentration in the light environment by 1–2 orders of magnitude[60]. AOA may be more sensitive to light owing to insufficient antioxidant capacity. Thus AOB occupied almost all of the ammonia oxidizers in seawater from 0–100 m depth[34].
In summary, AOA have difficulty surviving in eutrophic surface seawater, but the dark, deep sea serves as a vast living space for AOA and protects them from the inhibition of high pH, high dissolved oxygen, and high ROS.
3 The basis for deep-sea adaptation of AOAAlthough AOA in the deep sea is protected from adverse conditions such as high pH and photooxidation, the deep sea, in general, is a harsh environment characterized with high pressure, low temperature, low nutrient availability for life. Therefore, it is likely that the AOA have specific basic characteristics, enabling them to adapt to environmental changes in expanding from land to the deep sea (Figure 2). Besides, evolution and LGT also increase their adaptation.
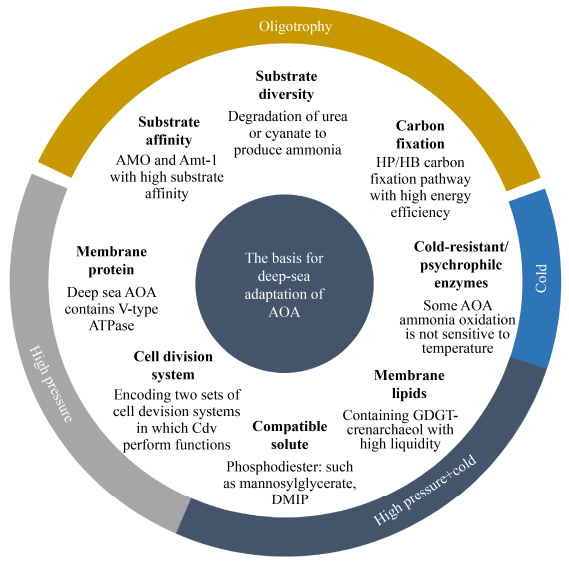
|
| Figure 2 Potential adaptation of AOA to the deep-sea environment. |
3.1 Sufficient membrane fluidity
Lipids are susceptible to stress. On average, they represent an order of magnitude more compressible than proteins. Many deep-sea microorganisms respond to HHP by regulating the composition of membranes. Such as producing higher content of monounsaturated or polyunsaturated fatty acid (M/PUFA), terminal branched fatty acids (TBFA), and fatty acids of short acyl chain length[62–63]. These changes increase membrane fluidity and avoid curing under high pressure and low-temperature conditions.
The membrane lipids of archaea differ from the bilayer membrane of bacteria, but rather glycerol dibiphytanyl glycerol tetraether membrane lipids (GDGTs) containing different core groups. They are monolayer phospholipids, consisting of a core membrane lipid and a polar head group. AOA all contain a high abundance of Thaumarchaeota- specific crenarchaeol core GDGTs. These membrane lipids are ubiquitous in the ocean[64]. Its molecular structure contains one cyclohexane and four cyclopentane rings, similar to the GDGT-4 synthesized by (super) thermophilic Crenarchaeota, but its internal cyclization double phytoalkane hydrocarbon chain forms a cyclohexane. Thus, the membrane fluidity is improved, being beneficial for AOA to adapt to the extreme environment in the deep sea[65]. The composition of GDGTs of different taxa of AOA and analogous AOA in altered metabolic states is highly variable[64], suggesting that the GDGTs have an essential role in the physiological function of AOA. For example, Ca. Nitrosotalea devanaterra has a high abundance of GDGT-4[66], resulting in their lower membrane permeability, giving them the ability to grow in low pH environments. GDGT-X is currently found only in Ca. Nitrososphaera gargensis Ga9.2, which is abundant in membranes, may be related to the thermophilic nature of Nitrososphaerales (Group I.1b)[67]. However, there is a lack of researches on GDGTs of AOA from the deep sea, the relationship among GDGTs composition, function, and deep-sea environment, and whether there is an undiscovered type of GDGTs still need further research and discussion.
3.2 Ability to maintain enzyme activityTemperature is the main factor in determining the chemical reaction rate. The psychrophilic enzyme with high specific activity is often an order of magnitude higher in microorganisms living in low temperatures than mesophilic microorganisms[43]. The generally accepted hypothesis is that psychrophilic enzymes increase the flexibility of their structures to compensate for the "freezing effect" in cold habitats[68]. Although the structure of psychrophilic proteins is generally similar to their mesophilic or thermophilic homologous proteins, the active left of psychrophilic proteins is more accessible. Specifically: reducing the number of electrostatic interactions, hydrogen bonds, and hydrophobic interactions, decreasing subunit interactions, increasing interactions with solvents, reducing non-polar structures in the core, increasing the contact between non-polar residues and solvents, lessening the combination of cofactors, aggregating glycine residues and reducing proline and arginine contents[69]. It shows that the high catalytic constant (kcat) of psychrophilic enzymes at low temperature is due to the decrease of activation enthalpy and activation entropy, which causes the enzyme-substrate complexes to display a broader conformation[70]. Therefore, enhancing the flexibility of psychrophilic enzymes also reduces the affinity between enzymes and substrates[71]. High pressures and low temperatures increase protein hydration, thus increasing the osmotic pressure inhibits protein expansion and exposing more hydrated surface area, which helps maintain a more compact structure and enzyme activity[72].
Although the optimum temperature for ammonia oxidation of AOA is generally greater than 20 ℃, some studies based on in situ measurements have shown that temperature has no significant effect on the AOA ammonia oxidation activity in the range of 8–20 ℃[73]. This indicates that there may be a particular type of AOA whose AMO can withstand low temperatures. Enhancing osmotic pressure reduces hydration and helps maintain protein activity under high pressure and low temperature. The presence of putative mannosy-l-3-phosphoglycerate synthase in the genome and proteome of terrestrial-derived Nitrososphaerales-AOA indicates that this type of AOA can synthesize compatible solute mannosylglycerate from mannose-6-phosphate[74]. Based on genomic analysis, almost all deep-sea AOA have the potential to produce di-myo- inositol-phosphate (DMIP), which can act as an osmotic pressure regulator to deal with HHP and low temperature in the deep sea[75].
3.3 Stability of DNA structure and functionHigh pressure and low temperature make DNA melting difficult, affecting DNA replication and transcription. Genetic studies have shown that the DNA recombination repair system also plays a part in HHP adaptation. Many non-piezophilic microorganisms become filamentous at high pressures that allow cell growth, probably because cell division is more sensitive to stress than cell growth[76]. Similarly, piezophilic bacteria are filamentous when grown at pressures below or above their optimal pressure[76]. Proteins involved in cell division may be susceptible to changes in pressure. FtsZ is a kind of tubulin-like GTP hydrolyzed protein that is extensively present in prokaryotes. They control the division process of prokaryotic cells. In the early stage of the separation, FtsZ aggregates to form a ring in the left of the cell. In the cell of Escherichia coli incubated under high pressure, the FtsZ ring was almost absent, but it formed quickly after decompression[77].
AOA encode two replication systems, FtsZ and Cdv, but only the Cdv system controls the splitting process[78]. Members of Euryarchaeota contain FtsZ protein, while Crenarchaeota lacks FtsZ but contains Cdv protein. The sequenced AOA were all found to encode FtsZ and Cdv, indicating that AOA have evolved/obtained these two systems very early. Heterologous expression studies have shown that the Cdv protein of Nitrosopumilus maritimus SCM1 forms a stable complex in yeast compared to the rapid turnover of the FtsZ protein in E. coli. This may be related to the slow division cycle or oligotrophic living environments[79]. However, the characteristics of the Cdv of deep or shallow sea AOA under high pressure are still unclear.
3.4 Maintaining the function of membrane proteinsThe pressure of 1×108 Pa causes a decrease in lipid fluidity and reversible conformational changes in transmembrane proteins resulting in disturbance of Na+/K+-ATPase function[80]. In comparison, the genomes of hadal AOA have two sets of ATPase (A-type and V-type). The V-type ATPase is discovered in all deep-sea AOA genomes. Due to the ability to pump out protons, V-type ATPase is also an acidophilic basis of Ca. Nitrosotalea. Hydrostatic pressure up to 2×107 Pa has completely inhibited the growth of shallow-sea AOA Nitrosopumilus maritimus SCM1 (without V-type ATPase gene)[75]. It shows that pumping out protons by V-type ATPase when energy is sufficient is one possible mechanism for high-pressure adaptability of deep-sea AOA. Still, more AOA strain need to be isolated and cultured from the deep sea to conclusively confirm the role of V-type ATPase in adaptation to high pressure.
3.5 Adaptation to oligotrophic environmentAlthough the total stoichiometry of AOA ammonia oxidation is equal to AOB, AOA have a very high affinity for ammonia. For instance, N. maritimus SCM1 oxidized unionized ammonia (NH3) with the half-saturation constant (km) of about 3 nmol/L[81] (calculated by Emerson et al.'s formulas[82]). In addition to being oxidized on the outer side of the cytomembrane as an energy source, ammonia also serves as a substrate for synthesizing nitrogen-containing compounds, which require extracellular ammonium to be transported into cells through ammonium transporters (Amt). Amt transporters encoded in AOA genomes define two separate lineages, Amt-1 and Amt-2, while marine AOA have Amt-1 with high substrate affinity[83]. The superior affinity of AMO and Amt transporter indicates that AOA are advantages in the competition for ammonia nitrogen in oligotrophic environments.
AOA perform carbon fixation through a modified hydroxypropionate/hydroxybutyrate (HP/HB) cycle with the highest energy efficiency among aerobic autotrophic pathways[84], making it possible for them to multiply in low energy conditions.
Soluble organic nitrogen, such as urea and cyanate, is prevalent in the oceans, with urea concentrations roughly similar to ammonia and cyanate about an order of magnitude lower. AOA can degrade urea and cyanate, thus supplementing nitrogen and energy requirements under nitrogen-limited conditions[85]. The urease is often encoding in the marine AOA genomes[9, 11, 36]. However, the gene of cyanase is only found in Nitrososphaera gargensis Ga9.2 derived from terrestrial hot springs[86], even though the pure culture of Nitrosopumilus maritimus SCM1 without cyanase still utilizes cyanate as a substrate for ammonia oxidation[85].
Since ammonia oxidation in the deep sea is limited by cold, oligotrophic, and other extreme conditions. The gtsABC gene with glucose uptake function has been identified in the marine δ group (based on 16S rRNA gene classification)[36]. Moreover, the AOA genome encodes various transporters, indicating that multiple organic matters may be utilized as precursors[83]. Some strains of Nitrosopumilus stimulated by α-keto acid, showing potential mixotrophy[9]. However, the promotion of marine AOA by α-keto acids finally shown to act as a ROS scavenger. Through the 13C isotope substrate labeling experiments, Nitrosopumilus sp. DDS1 and Nitrososphaera viennensis EN76 stimulated to grow by the organic matter are strictly autotrophic microorganisms[59]. The terrestrial AOA, Ca. Nirosocosmicus exaquare G61 has a robust antioxidant system and various organic substances stimulating its growth in enrichment, even if some of them do not eliminate ROS[28]. Ca. Nitrosocosmicus arcticus Kfb, originating from Arctic soil, grew faster at low temperatures (4–8 ℃) than at moderate temperatures (20–28 ℃); Unexpectedly, nitrite production was not detected unless increasing incubation temperature[17]. This evidence suggests that Ca. Nitrosocosmicus might have diverse metabolic capabilities. However, there is still a lack of culture-based experiments to study the trophic type of deep-sea AOA.
4 The role of AOA in deep-sea environmentThe carbon cycle in the ocean plays a fundamental role in the habitability of the planet. Organic and inorganic carbon is transformed into each other regulating the atmospheric CO2 concentration. The surface phytoplankton collect atmospheric CO2 through photosynthesis. Then, they export particulate organic carbon (POC) or dissolved organic carbon (DOC) to the deep sea[87], that is the biological pumps.
However, organic carbon reaching the seafloor represents only about 0.3% of the primary productivity of the ocean[88]. Heterotrophic microorganisms consume the vast majority of them to produce CO2 during the sedimentation process. Thus, organic carbon exists in the oceans mainly as recalcitrant dissolved organic carbon (RDOC) that is difficult to degrade[89].
RDOC as colossal carbon sinks is mainly generated by microbial degradation, synthesis, secretion. These processes release CO2 and inorganic nitrogen and phosphorus[90], that is, the role of microbial carbon pump (MCP). AOA and other chemoautotrophic microorganisms use inorganic carbon and nutrient salts to synthesize organic matter in the deep sea, recover CO2 produced by the MCP and provide new organic carbon for the MCP, enhancing the effect of carbon sequestration.
Primary producers use light or chemical energy to convert CO2 to organic metabolites while changing the stoichiometric composition of the cell to affect other element cycles, such as the nitrogen cycle[53]. Nitrification is an essential segment of the global nitrogen cycle. For a long time, researchers consider AOB is the only ammonia oxidizer group, but the discovery of AOA has led to a reassessment of the ammonia oxidation process. As a central process in the nitrogen cycle, nitrification linking the biological processes of nitrogen fixation and nitrogen loss (denitrification and anaerobic ammonia oxidation)[91]. It consists of two steps: ammonia oxidation and nitrite oxidation. Nitrification energizes two chemoautotrophs (ammonia oxidizer and nitrite oxidizer), driving the coupling between reduced inorganic nitrogen and newly generated organic carbon.
AOA is much more abundant in the deep sea than AOB[34, 92]. In situ studies have shown that AOA are active in the marine environment[93–96]. Despite the ammonia oxidation rate of AOA is very low[5], the enormous biomass means that the contribution of marine AOA to the carbon and nitrogen cycle of the Earth is considerable. According to estimates, deep-sea nitrifying microorganisms can fix 1×1013–2×1013 moles of carbon and oxidize 1×1014–2×1014 moles of ammonia per year in the deep sea[97], which has a profound impact on climate change. Figure 3 macroscopically illustrates the involvement of AOA in the marine geochemical cycle. Settled nitrogenous organic matters are decomposed and mineralized by heterotrophic microorganisms, thereby releasing ammonia, urea, cyanate, which serve as energy sources and biosynthetic substrates for AOA. Nitrite-oxidizing bacteria (NOB) use nitrite produced by AOA as an oxidation substrate, and these two groups of nitrifier provide oxidized nitrogen for denitrification. AOA synthesize polysaccharides, lipids, nitrogenous organics, and other metabolites from inorganic carbon using the energy generated by ammonia oxidation (Figure 4). Heterotrophic organisms can use these primary metabolites as important energy support for deep-sea ecosystems. The refractory components dissolve in seawater or settle to the seafloor and no longer enter the atmosphere. Thus, AOA may play a key role in the process of carbon fixation and sequestration in the ocean as an essential complement to MCP.

|
| Figure 3 Ocean carbon cycle and the nitrogen cycle in which AOA is primarily involved. POM: particulate organic matter; DOM: dissolved organic matter; NFB: nitrogen-fixing bacteria; AOB: ammonia-oxidizing bacteria; NOB: nitrite-oxidizing bacteria; AOA: ammonia-oxidizing archaea); DB: denitrifying bacteria; HB: heterotrophic bacteria. |

|
| Figure 4 Carbon and nitrogen metabolism pathway related to the geochemical cycle of marine AOA. Amt: ammonium transporter; Amo: ammonia monooxygenase; HURM: hydroxylamine ubiquinone redox module; NIR: nitrite reductase; pcy: plastocyanin; III: complex III; IV: complex IV; V: ATPase; GTGDs: glycerol dibiphytanyl glycerol tetraether lipids. |
AOA are also adaptable to toxic deep-sea environments, such as surviving in hydrothermal vents[98] and cold spring sediments[99] containing reduced sulfur, which is different from AOB sensitive to H2S[100]. Also, co-culture with sulfur-oxidizing bacteria (SOB) in thiosulfate-containing media can promote the growth of AOA (probably SOB consumes the extra oxygen)[12]. It indicated that AOA is responsible for primary production based on ammonia oxidation and may significantly influence other primary production processes such as sulfur oxidation.
A great deal of research has been conducted in revealing the role of AOA in the marine geochemical cycle. However, whether AOA consume organic matter as energy sources, in addition to autotrophic ammonia oxidation, remains inconclusive. In addition to death or lysis by phages, AOA secrete DOC, which can be utilized by heterotrophic microorganisms, including amino acids, thymine, B vitamins[101]. The secreted DOC by AOA account for only 0.08%–1.05% of carbon requirement of heterotrophic prokaryotes[102]. They are crucial to some AOA-related auxotrophic microorganisms, suggesting that there may be close cooperation between AOA and some heterotrophic microorganisms.
5 PerspectivesGiven the irreplaceable role of marine AOA in the carbon and nitrogen cycles, the study of the metabolic properties of AOA also helps to improve our understanding on the geochemical cycles in which AOA participates. Unfortunately, at present, there is still limited knowledge on the metabolism of AOA, such as the processes of energy sources other than ammonia (e.g., urea and cyanate), energy metabolism (e.g., hydroxylamine oxidation), the composition of synthesized primary products, and the regulatory mechanism of carbon and nitrogen metabolism process (Figure 4).
As AOA expands its habitats into the deep sea, high species diversity has developed along with evolution and LGT along with environmental pressure. Culture-based research of AOA forms the core of our knowledge on this type of microorganisms and promotes understanding of the biochemical cycle in which they participate. AOA strains from different sources show significant differences in genome and metabolic functions. However, the cultured AOA only belong to a few branches of global AOA evolution trees (Figure 1), and a large number of uncultured AOAs from different environments are waiting for in-depth study.
Isolated AOA strains are crucial to confirming the physiological characteristics of AOA from different water depths or different groups. However, AOA are difficult-to-culture microorganisms and often require long-term enrichment to obtain cultures[103]. Many important findings are based on culture, such as the determination of the function[1], kinetics of AOA ammonia oxidation[5], a putative autotrophic ammonia oxidation model[104], α-keto acid facilitates antioxidation of AOA[59], and the identification of carbon fixation cycle[84]. Metagenomic techniques provide direct access to the genomes of microorganisms in the environment to predict their metabolic properties and have broad applications in mining the genetic resources of uncultured microorganisms. Auxotype determines how AOA participates in the carbon and nitrogen cycle, but in situ surveys and genomic-based analyses do not provide conclusive evidence for the auxotype of deep-sea AOA. Ammonia oxidation kinetics are also crucial to re-estimating global oceanic nitrogen fluxes. Current studies are mainly based on a few shallow-sea AOA strains. The differences in physiological characteristics between shallow and deep-sea AOA may dramatically impact the calculation of oceanic nitrogen fluxes. Culture-based methods in combine with in situ geochemical analysis will provide more reliable data to approaching a precise evaluation on the ecological function of these microorganisms in terms of driving the nitrogen cycling in deep sea.
Apparently, AOA has long generation time, potential piezophilic properties, and interactions with other microorganisms; its growth is inhibited by ammonia[24]and ROS[59]. All these features lead us to develop novel approaches for deep-sea AOA cultivation. For example, we can use deep-sea simulation technology to achieve cultivation conditions that are similar to their natural habitats. Besides, a modified medium matching their metabolic potential based on the genetic analysis is also recommended. For example, a continuous supplementation with very low concentrations of ammonia nitrogen and ROS scavengers in a facility that can perform under high pressure and low temperatures. Studying physiological properties and metabolic processes of deep-sea AOA on this basis will play a major part in illuminating their contribution to the geochemical cycle, adaptation, and biological evolution of deep-sea life. The isolation of deep-sea AOA will also be beneficial to the study of the relationship between oligotrophy and high-pressure adaptation, which is helpful for us to understand the living strategy in deep biosphere.
| [1] | Könneke M, Bernhard AE, de la Torre JR, Walker CB, Waterbury JB, Stahl DA. Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature, 2005, 437(7058): 543-546. DOI:10.1038/nature03911 |
| [2] | Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic crenarchaeota: proposal for a third archaeal Phylum, the Thaumarchaeota. Nature Reviews Microbiology, 2008, 6(3): 245-252. DOI:10.1038/nrmicro1852 |
| [3] | Prosser JI, Nicol GW. Relative contributions of Archaea and bacteria to aerobic ammonia oxidation in the environment. Environmental Microbiology, 2008, 10(11): 2931-2941. DOI:10.1111/j.1462-2920.2008.01775.x |
| [4] | Kuypers MMM, Marchant HK, Kartal B. The microbial nitrogen-cycling network. Nature Reviews Microbiology, 2018, 16(5): 263-276. DOI:10.1038/nrmicro.2018.9 |
| [5] | Martens-Habbena W, Berube PM, Urakawa H, de la Torre JR, Stahl DA. Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature, 2009, 461(7266): 976-979. DOI:10.1038/nature08465 |
| [6] | Ren ML, Feng XY, Huang YJ, Wang H, Hu Z, Clingenpeel S, Swan BK, Fonseca MM, Posada D, Stepanauskas R, Hollibaugh JT, Foster PG, Woyke T, Luo HW. Phylogenomics suggests oxygen availability as a driving force in Thaumarchaeota evolution. The ISME Journal, 2019, 13(9): 2150-2161. DOI:10.1038/s41396-019-0418-8 |
| [7] | Kerou M, Schleper C. Nitrososphaeria. Bergey's Manual of Systematics of Archaea and Bacteria. New York: John Wiley & Sons, 2016. |
| [8] | Eloy Alves RJ, Minh BQ, Urich T, von Haeseler A, Schleper C. Unifying the global phylogeny and environmental distribution of ammonia-oxidising Archaea based on amoA genes. Nature Communications, 2018, 9: 1517. DOI:10.1038/s41467-018-03861-1 |
| [9] | Qin W, Amin SA, Martens-Habbena W, Walker CB, Urakawa H, Devol AH, Ingalls AE, Moffett JW, Armbrust EV, Stahl DA. Marine ammonia-oxidizing archaeal isolates display obligate mixotrophy and wide ecotypic variation. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(34): 12504-12509. DOI:10.1073/pnas.1324115111 |
| [10] | Santoro AE, Dupont CL, Richter RA, Craig MT, Carini P, McIlvin MR, Yang Y, Orsi WD, Moran DM, Saito MA. Genomic and proteomic characterization of "Candidatus Nitrosopelagicus brevis": an ammonia-oxidizing archaeon from the open ocean. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(4): 1173-1178. DOI:10.1073/pnas.1416223112 |
| [11] | Bayer B, Vojvoda J, Offre P, Alves RJE, Elisabeth NH, Garcia JA, Volland JM, Srivastava A, Schleper C, Herndl GJ. Physiological and genomic characterization of two novel marine thaumarchaeal strains indicates niche differentiation. The ISME Journal, 2016, 10(5): 1051-1063. DOI:10.1038/ismej.2015.200 |
| [12] | Park BJ, Park SJ, Yoon DN, Schouten S, Sinninghe Damsté JS, Rhee SK. Cultivation of autotrophic ammonia-oxidizing Archaea from marine sediments in coculture with sulfur-oxidizing bacteria. Applied and Environmental Microbiology, 2010, 76(22): 7575-7587. DOI:10.1128/AEM.01478-10 |
| [13] | Hatzenpichler R, Lebedeva EV, Spieck E, Stoecker K, Richter A, Daims H, Wagner M. A moderately thermophilic ammonia-oxidizing crenarchaeote from a hot spring. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(6): 2134-2139. DOI:10.1073/pnas.0708857105 |
| [14] | Lebedeva EV, Hatzenpichler R, Pelletier E, Schuster N, Hauzmayer S, Bulaev A, Grigor'eva NV, Galushko A, Schmid M, Palatinszky M, Le Paslier D, Daims H, Wagner M. Enrichment and genome sequence of the group I.1a ammonia-oxidizing archaeon "Ca. Nitrosotenuis uzonensis" representing a clade globally distributed in thermal habitats.. PLoS ONE, 2013, 8(11): e80835. DOI:10.1371/journal.pone.0080835 |
| [15] | Abby SS, Melcher M, Kerou M, Krupovic M, Stieglmeier M, Rossel C, Pfeifer K, Schleper C. Candidatus Nitrosocaldus cavascurensis, an ammonia oxidizing, extremely thermophilic archaeon with a highly mobile genome. Frontiers in Microbiology, 2018, 9: 28. DOI:10.3389/fmicb.2018.00028 |
| [16] | Daebeler A, Herbold C, Vierheilig J, Sedlacek CJ, Pjevac P, Albersten M, Kirkegaard RH, de la Torre JR, Daims H, Wagner M. Cultivation and genomic analysis of Candidatus Nitrosocaldus islandicus, a novel obligately thermophilic ammonia-oxidizing Thaumarchaeon. bioRxiv, 2017. DOI:10.1101/235028 |
| [17] | Alves RJE, Kerou M, Zappe A, Bittner R, Abby SS, Schmidt HA, Pfeifer K, Schleper C. Ammonia oxidation by the arctic terrestrial thaumarchaeote Candidatus Nitrosocosmicus arcticus is stimulated by increasing temperatures. Frontiers in Microbiology, 2019, 10: 1571. DOI:10.3389/fmicb.2019.01571 |
| [18] | Tourna M, Stieglmeier M, Spang A, Konneke M, Schintlmeister A, Urich T, Engel M, Schloter M, Wagner M, Richter A, Schleper C. Nitrososphaera viennensis, an ammonia oxidizing archaeon from soil. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(20): 8420-8425. DOI:10.1073/pnas.1013488108 |
| [19] | Jung MY, Park SJ, Min D, Kim JS, Rijpstra WIC, Sinninghe Damsté JS, Kim GJ, Madsen EL, Rhee SK. Enrichment and characterization of an autotrophic ammonia-oxidizing archaeon of mesophilic crenarchaeal group I.1a from an agricultural soil.. Applied and Environmental Microbiology, 2011, 77(24): 8635-8647. DOI:10.1128/AEM.05787-11 |
| [20] | Lehtovirta-Morley LE, Stoecker K, Vilcinskas A, Prosser JI, Nicol GW. Cultivation of an obligate acidophilic ammonia oxidizer from a nitrifying acid soil. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(38): 15892-15897. DOI:10.1073/pnas.1107196108 |
| [21] | Kim JG, Jung MY, Park SJ, Rijpstra WIC, Sinninghe Damsté JS, Madsen EL, Min D, Kim JS, Kim GJ, Rhee SK. Cultivation of a highly enriched ammonia-oxidizing archaeon of thaumarchaeotal group I.1b from an agricultural soil.. Environmental Microbiology, 2012, 14(6): 1528-1543. DOI:10.1111/j.1462-2920.2012.02740.x |
| [22] | Jung MY, Park SJ, Kim SJ, Kim JG, Sinninghe Damsté JS, Jeon CO, Rhee SK. A mesophilic, autotrophic, ammonia-oxidizing archaeon of thaumarchaeal group I.1a cultivated from a deep oligotrophic soil horizon.. Applied and Environmental Microbiology, 2014, 80(12): 3645-3655. DOI:10.1128/AEM.03730-13 |
| [23] | Lehtovirta-Morley LE, Ross J, Hink L, Weber EB, Gubry-Rangin C, Thion C, Prosser JI, Nicol GW. Isolation of 'Candidatus Nitrosocosmicus franklandus', a novel ureolytic soil archaeal ammonia oxidiser with tolerance to high ammonia concentration. FEMS Microbiology Ecology, 2016, 92(5): fiw057. DOI:10.1093/femsec/fiw057 |
| [24] | Liu LT, Liu MF, Jiang YM, Lin WT, Luo JF. Production and excretion of polyamines to tolerate high ammonia, a case study on soil ammonia-oxidizing archaeon "Candidatus Nitrosocosmicus agrestis". mSystems, 2021, 6(1): e01003-20. |
| [25] | Sauder LA, Engel K, Lo CC, Chain P, Neufeld JD. "Candidatus Nitrosotenuis aquarius, " an ammonia-oxidizing archaeon from a freshwater aquarium biofilter. Applied and Environmental Microbiology, 2018, 84(19): e01430-18. |
| [26] | Li YY, Ding K, Wen XH, Zhang B, Shen B, Yang YF. A novel ammonia-oxidizing archaeon from wastewater treatment plant: Its enrichment, physiological and genomic characteristics. Scientific Reports, 2016, 6: 23747. DOI:10.1038/srep23747 |
| [27] | Chen HY, Yue YY, Jin WB, Zhou X, Wang QL, Gao SH, Xie GJ, Du S, Tu RJ, Han SF, Guo KX. Enrichment and characteristics of ammonia-oxidizing archaea in wastewater treatment process. Chemical Engineering Journal, 2017, 323: 465-472. DOI:10.1016/j.cej.2017.04.130 |
| [28] | Sauder LA, Albertsen M, Engel K, Schwarz J, Nielsen PH, Wagner M, Neufeld JD. Cultivation and characterization of Candidatus Nitrosocosmicus exaquare, an ammonia-oxidizing archaeon from a municipal wastewater treatment system. The ISME Journal, 2017, 11(5): 1142-1157. DOI:10.1038/ismej.2016.192 |
| [29] | Ochsenreiter T, Selezi D, Quaiser A, Bonch-Osmolovskaya L, Schleper C. Diversity and abundance of Crenarchaeota in terrestrial habitats studied by 16S RNA surveys and real time PCR. Environmental Microbiology, 2003, 5(9): 787-797. DOI:10.1046/j.1462-2920.2003.00476.x |
| [30] | Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C. Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature, 2006, 442(7104): 806-809. DOI:10.1038/nature04983 |
| [31] | Shen JP, Zhang LM, Zhu YG, Zhang JB, He JZ. Abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea communities of an alkaline sandy loam. Environmental Microbiology, 2008, 10(6): 1601-1611. DOI:10.1111/j.1462-2920.2008.01578.x |
| [32] | Zhang LM, Hu HW, Shen JP, He JZ. Ammonia-oxidizing archaea have more important role than ammonia-oxidizing bacteria in ammonia oxidation of strongly acidic soils. The ISME Journal, 2012, 6(5): 1032-1045. DOI:10.1038/ismej.2011.168 |
| [33] | Karner MB, DeLong EF, Karl DM. Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature, 2001, 409(6819): 507-510. DOI:10.1038/35054051 |
| [34] | Nunoura T, Takaki Y, Hirai M, Shimamura S, Makabe A, Koide O, Kikuchi T, Miyazaki J, Koba K, Yoshida N, Sunamura M, Ken TK. Hadal biosphere: Insight into the microbial ecosystem in the deepest ocean on Earth. Proceedings of the National Academy of Sciences of the United States of America, 2015, 112(11): E1236. |
| [35] | Nunoura T, Nishizawa M, Hirai M, Shimamura S, Harnvoravongchai P, Koide O, Morono Y, Fukui T, Inagaki F, Miyazaki J, Takaki Y, Ken TK. Microbial diversity in sediments from the bottom of the challenger deep, the Mariana trench. Microbes and Environments, 2018, 33(2): 186-194. DOI:10.1264/jsme2.ME17194 |
| [36] | Wang Y, Huang JM, Cui GJ, Nunoura T, Takaki Y, Li WL, Li J, Gao ZM, Ken TK, Zhang AQ, Stepanauskas R. Genomics insights into ecotype formation of ammonia-oxidizing archaea in the deep ocean. Environmental Microbiology, 2019, 21(2): 716-729. DOI:10.1111/1462-2920.14518 |
| [37] | Fang JS, Zhang L, Bazylinski DA. Deep-sea piezosphere and piezophiles: geomicrobiology and biogeochemistry. Trends in Microbiology, 2010, 18(9): 413-422. DOI:10.1016/j.tim.2010.06.006 |
| [38] | Mozhaev VV, Heremans K, Frank J, Masson P, Balny C. High pressure effects on protein structure and function. Proteins: Structure, Function, and Bioinformatics, 1996, 24(1): 81-91. DOI:10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R |
| [39] | Balny C, Masson P, Heremans K. High pressure effects on biological macromolecules: from structural changes to alteration of cellular processes. Biochimica et Biophysica Acta, 2002, 1595(1/2): 3-10. |
| [40] | Meersman F, Heremans K. High hydrostatic pressure effects in the biosphere: from molecules to microbiology. //High-Pressure Microbiology. Washington, DC, USA: ASM Press, 2014: 1-17. |
| [41] | Winter R. Synchrotron X-ray and neutron small-angle scattering of lyotropic lipid mesophases, model biomembranes and proteins in solution at high pressure. Biochimica et Biophysica Acta: BBA-Protein Structure and Molecular Enzymology, 2002, 1595(1/2): 160-184. |
| [42] | Dubins DN, Lee A, MacGregor RB, Chalikian TV. On the stability of double stranded nucleic acids. Journal of the American Chemical Society, 2001, 123(38): 9254-9259. DOI:10.1021/ja004309u |
| [43] | Feller G, Gerday C. Psychrophilic enzymes: hot topics in cold adaptation. Nature Reviews Microbiology, 2003, 1(3): 200-208. DOI:10.1038/nrmicro773 |
| [44] | D'Amico S, Collins T, Marx JC, Feller G, Gerday C, Gerday C. Psychrophilic microorganisms: challenges for life. EMBO Reports, 2006, 7(4): 385-389. DOI:10.1038/sj.embor.7400662 |
| [45] | Hedges JI, Eglinton G, Hatcher PG, Kirchman DL, Arnosti C, Derenne S, Evershed RP, Kögel-Knabner I, de Leeuw JW, Littke R, Michaelis W, Rullkötter J. The molecularly-uncharacterized component of nonliving organic matter in natural environments. Organic Geochemistry, 2000, 31(10): 945-958. DOI:10.1016/S0146-6380(00)00096-6 |
| [46] | Aluwihare LI, Repeta DJ, Chen RF. Chemical composition and cycling of dissolved organic matter in the Mid-Atlantic Bight. Deep Sea Research Part Ⅱ: Topical Studies in Oceanography, 2002, 49(20): 4421-4437. DOI:10.1016/S0967-0645(02)00124-8 |
| [47] | Pantoja S, Sepúlveda J, González HE. Decomposition of sinking proteinaceous material during fall in the oxygen minimum zone off northern Chile. Deep Sea Research Part I: Oceanographic Research Papers, 2004, 51(1): 55-70. DOI:10.1016/j.dsr.2003.09.005 |
| [48] | Hügler M, Sievert SM. Beyond the Calvin cycle: autotrophic carbon fixation in the ocean. Annual Review of Marine Science, 2011, 3: 261-289. DOI:10.1146/annurev-marine-120709-142712 |
| [49] | Herndl GJ, Reinthaler T. Microbial control of the dark end of the biological pump. Nature Geoscience, 2013, 6(9): 718-724. DOI:10.1038/ngeo1921 |
| [50] | Karl DM, Church MJ, Dore JE, Letelier RM, Mahaffey C. Predictable and efficient carbon sequestration in the North Pacific Ocean supported by symbiotic nitrogen fixation. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(6): 1842-1849. DOI:10.1073/pnas.1120312109 |
| [51] | Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P, Strous M, Teira E, Herndl GJ, Middelburg JJ, Schouten S, Sinninghe Damste JS. Archaeal nitrification in the ocean. PNAS, 2006, 103(33): 12317-12322. DOI:10.1073/pnas.0600756103 |
| [52] | Karl DM. Microbial oceanography: paradigms, processes and promise. Nature Reviews Microbiology, 2007, 5(10): 759-769. DOI:10.1038/nrmicro1749 |
| [53] | Zehr JP, Kudela RM. Nitrogen cycle of the open ocean: from genes to ecosystems. Annual Review of Marine Science, 2011, 3: 197-225. DOI:10.1146/annurev-marine-120709-142819 |
| [54] | Baltar F, Arístegui J, Gasol JM, Yokokawa T, Herndl GJ. Bacterial versus archaeal origin of extracellular enzymatic activity in the northeast Atlantic deep waters. Microbial Ecology, 2013, 65(2): 277-288. DOI:10.1007/s00248-012-0126-7 |
| [55] | Dekas AE, Fike DA, Chadwick GL, Green-Saxena A, Fortney J, Connon SA, Dawson KS, Orphan VJ. Widespread nitrogen fixation in sediments from diverse deep-sea sites of elevated carbon loading. Environmental Microbiology, 2018, 20(12): 4281-4296. DOI:10.1111/1462-2920.14342 |
| [56] | Garcia HE, Weathers KW, Paver CR, Smolyar I, Boyer TP, Locarnini RA, Zweng MM, Mishonov AV, Baranova OK, Seidov D, Reagan JR. World Ocean Atlas 2018, Volume 4:Dissolved onorganic nutrients (phosphate, nitrate and nitrate+nitrite, silicate). NOAA Atlas NESDIS 84, 2019: 35. |
| [57] | Garcia HE, Weathers KW, Paver CR, Smolyar I, Boyer TP, Locarnini RA, Zweng MM, Mishonov AV, Baranova OK, Seidov D, Reagan JR. World Ocean Atlas 2018, Volume 3:Dissolved oxygen, apparent oxygen utilization, and dissolved oxygen saturation. NOAA Atlas NESDIS 83, 2019: 38. |
| [58] | Qin W, Heal KR, Ramdasi R, Kobelt JN, Martens-Habbena W, Bertagnolli AD, Amin SA, Walker CB, Urakawa H, Könneke M, Devol AH, Moffett JW, Armbrust EV, Jensen GJ, Ingalls AE, Stahl DA. Nitrosopumilus maritimus gen. nov., sp. nov., Nitrosopumilus cobalaminigenes sp. nov., Nitrosopumilus oxyclinae sp. nov., and Nitrosopumilus ureiphilus sp. nov., four marine ammonia-oxidizing archaea of the Phylum Thaumarchaeota. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(12): 5067-5079. DOI:10.1099/ijsem.0.002416 |
| [59] | Kim JG, Park SJ, Sinninghe Damsté JS, Schouten S, Rijpstra WIC, Jung MY, Kim SJ, Gwak JH, Hong H, Si OJ, Lee S, Madsen EL, Rhee SK. Hydrogen peroxide detoxification is a key mechanism for growth of ammonia-oxidizing Archaea. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(28): 7888-7893. DOI:10.1073/pnas.1605501113 |
| [60] | Tolar BB, Powers LC, Miller WL, Wallsgrove NJ, Popp BN, Hollibaugh JT. Ammonia oxidation in the ocean can be inhibited by nanomolar concentrations of hydrogen peroxide. Frontiers in Marine Science, 2016, 3: 237. |
| [61] | Zhang DN, Yan SW, Song WH. Photochemically induced formation of reactive oxygen species (ROS) from effluent organic matter. Environmental Science & Technology, 2014, 48(21): 12645-12653. |
| [62] | Delong EF, Yayanos AA. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Applied and Environmental Microbiology, 1986, 51(4): 730-737. DOI:10.1128/aem.51.4.730-737.1986 |
| [63] | Yano Y, Nakayama A, Yoshida K. Distribution of polyunsaturated Fatty acids in bacteria present in intestines of deep-sea fish and shallow-sea poikilothermic animals. Applied and Environmental Microbiology, 1997, 63(7): 2572-2577. DOI:10.1128/aem.63.7.2572-2577.1997 |
| [64] | Pitcher A, Hopmans EC, Mosier AC, Park SJ, Rhee SK, Francis CA, Schouten S, Sinninghe Damsté JS. Core and intact polar glycerol dibiphytanyl glycerol tetraether lipids of ammonia-oxidizing archaea enriched from marine and estuarine sediments. Applied and Environmental Microbiology, 2011, 77(10): 3468-3477. DOI:10.1128/AEM.02758-10 |
| [65] | Damsté JSS, Schouten S, Hopmans EC, van Duin ACT, Geenevasen JAJ. Crenarchaeol: the characteristic core glycerol dibiphytanyl glycerol tetraether membrane lipid of cosmopolitan pelagic crenarchaeota. Journal of Lipid Research, 2002, 43(10): 1641-1651. DOI:10.1194/jlr.M200148-JLR200 |
| [66] | Lehtovirta-Morley LE, Sayavedra-Soto LA, Gallois N, Schouten S, Stein LY, Prosser JI, Nicol GW. Identifying potential mechanisms enabling acidophily in the ammonia-oxidizing archaeon "Candidatus Nitrosotalea devanaterra". Applied and Environmental Microbiology, 2016, 82(9): 2608-2619. DOI:10.1128/AEM.04031-15 |
| [67] | Pitcher A, Rychlik N, Hopmans EC, Spieck E, Irene C Rijpstra W, Ossebaar J, Schouten S, Wagner M. Crenarchaeol dominates the membrane lipids of Candidatus Nitrososphaera gargensis, a thermophilic Group I.1b Archaeon.. The ISME Journal, 2010, 4(4): 542-552. DOI:10.1038/ismej.2009.138 |
| [68] | Johns GC. Evolutionary convergence in adaptation of proteins to temperature: A4-lactate dehydrogenases of Pacific damselfishes (Chromis spp).. Molecular Biology and Evolution, 2003, 21(2): 314-320. DOI:10.1093/molbev/msh021 |
| [69] | Violot S, Aghajari N, Czjzek M, Feller G, Sonan GK, Gouet P, Gerday C, Haser R, Receveur-Bréchot V. Structure of a full length psychrophilic cellulase from Pseudoalteromonas haloplanktis revealed by X-ray diffraction and small angle X-ray scattering. Journal of Molecular Biology, 2005, 348(5): 1211-1224. DOI:10.1016/j.jmb.2005.03.026 |
| [70] | Lonhienne T, Gerday C, Feller G. Psychrophilic enzymes: revisiting the thermodynamic parameters of activation may explain local flexibility. Biochimica et Biophysica Acta: BBA-Protein Structure and Molecular Enzymology, 2000, 1543(1): 1-10. |
| [71] | Collins T, Meuwis MA, Stals I, Claeyssens M, Feller G, Gerday C. A novel family 8 xylanase, functional and physicochemical characterization. Journal of Biological Chemistry, 2002, 277(38): 35133-35139. DOI:10.1074/jbc.M204517200 |
| [72] | Gillett MB, Suko JR, Santoso FO, Yancey PH. Elevated levels of trimethylamine oxide in muscles of deep-sea gadiform teleosts: a high-pressure adaptation? Journal of Experimental Zoology, 1997, 279(4): 386-391. Journal of Experimental Zoology, 1997, 279(4): 386-391. DOI:10.1002/(SICI)1097-010X(19971101)279:4<386::AID-JEZ8>3.0.CO;2-K |
| [73] | Horak REA, Qin W, Schauer AJ, Virginia Armbrust E, Ingalls AE, Moffett JW, Stahl DA, Devol AH. Ammonia oxidation kinetics and temperature sensitivity of a natural marine community dominated by Archaea. The ISME Journal, 2013, 7(10): 2023-2033. DOI:10.1038/ismej.2013.75 |
| [74] | Kerou M, Offre P, Valledor L, Abby SS, Melcher M, Nagler M, Weckwerth W, Schleper C. Proteomics and comparative genomics of Nitrososphaera viennensis reveal the core genome and adaptations of archaeal ammonia oxidizers. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(49): E7937-E7946. DOI:10.1073/pnas.1601212113 |
| [75] | Wang BZ, Qin W, Ren Y, Zhou X, Jung MY, Han P, Eloe-Fadrosh EA, Li M, Zheng Y, Lu L, Yan X, Ji JB, Liu Y, Liu LM, Heiner C, Hall R, Martens-Habbena W, Herbold CW, Rhee SK, Bartlett DH, Huang L, Ingalls AE, Wagner M, Stahl DA, Jia ZJ. Expansion of Thaumarchaeota habitat range is correlated with horizontal transfer of ATPase operons. The ISME Journal, 2019, 13(12): 3067-3079. DOI:10.1038/s41396-019-0493-x |
| [76] | Bartlett DH. Pressure effects on in vivo microbial processes. Biochimica et Biophysica Acta: BBA-Protein Structure and Molecular Enzymology, 2002, 1595(1/2): 367-381. |
| [77] | Morild E. The theory of pressure effects on enzymes. Advances in Protein Chemistry, 1981, 34: 93-166. |
| [78] | Pelve EA, Lindås AC, Martens-Habbena W, de la Torre JR, Stahl DA, Bernander R. Cdv-based cell division and cell cycle organization in the thaumarchaeon Nitrosopumilus maritimus. Molecular Microbiology, 2011, 82(3): 555-566. DOI:10.1111/j.1365-2958.2011.07834.x |
| [79] | Ng KH, Srinivas V, Srinivasan R, Balasubramanian M. The Nitrosopumilus maritimus CdvB, but not FtsZ, assembles into polymers. Archaea, 2013, 2013: 1-10. |
| [80] | Kato M, Hayashi R, Tsuda T, Taniguchi K. High pressure-induced changes of biological membrane. European Journal of Biochemistry, 2002, 269(1): 110-118. DOI:10.1046/j.0014-2956.2002.02621.x |
| [81] | Kits KD, Sedlacek CJ, Lebedeva EV, Han P, Bulaev A, Pjevac P, Daebeler A, Romano S, Albertsen M, Stein LY, Daims H, Wagner M. Kinetic analysis of a complete nitrifier reveals an oligotrophic lifestyle. Nature, 2017, 549(7671): 269-272. DOI:10.1038/nature23679 |
| [82] | Emerson K, Russo RC, Lund RE, Thurston RV. Aqueous ammonia equilibrium calculations: effect of pH and temperature. Journal of the Fisheries Research Board of Canada, 1975, 32(12): 2379-2383. DOI:10.1139/f75-274 |
| [83] | Offre P, Kerou M, Spang A, Schleper C. Variability of the transporter gene complement in ammonia-oxidizing archaea. Trends in Microbiology, 2014, 22(12): 665-675. DOI:10.1016/j.tim.2014.07.007 |
| [84] | Könneke M, Schubert DM, Brown PC, Hügler M, Standfest S, Schwander T, Schada von Borzyskowski L, Erb TJ, Stahl DA, Berg IA. Ammonia-oxidizing archaea use the most energy-efficient aerobic pathway for CO2 fixation. Proceedings of the National Academy of Sciences of the United States of America, 2014, 111(22): 8239-8244. DOI:10.1073/pnas.1402028111 |
| [85] | Kitzinger K, Padilla CC, Marchant HK, Hach PF, Herbold CW, Kidane AT, Könneke M, Littmann S, Mooshammer M, Niggemann J, Petrov S, Richter A, Stewart FJ, Wagner M, Kuypers MMM, Bristow LA. Cyanate and urea are substrates for nitrification by Thaumarchaeota in the marine environment. Nature Microbiology, 2019, 4(2): 234-243. DOI:10.1038/s41564-018-0316-2 |
| [86] | Palatinszky M, Herbold C, Jehmlich N, Pogoda M, Han P, von Bergen M, Lagkouvardos I, Karst SM, Galushko A, Koch H, Berry D, Daims H, Wagner M. Cyanate as an energy source for nitrifiers. Nature, 2015, 524(7563): 105-108. DOI:10.1038/nature14856 |
| [87] | Worden AZ, Follows MJ, Giovannoni SJ, Wilken S, Zimmerman AE, Keeling PJ. Environmental science. Rethinking the marine carbon cycle: factoring in the multifarious lifestyles of microbes.. Science, 2015, 347(6223): 1257594. DOI:10.1126/science.1257594 |
| [88] | Zhang CL, Dang HY, Azam F, Benner R, Legendre L, Passow U, Polimene L, Robinson C, Suttle CA, Jiao NZ. Evolving paradigms in biological carbon cycling in the ocean. National Science Review, 2018, 5(4): 481-499. DOI:10.1093/nsr/nwy074 |
| [89] | Jiao NZ, Herndl GJ, Hansell DA, Benner R, Kattner G, Wilhelm SW, Kirchman DL, Weinbauer MG, Luo TW, Chen F, Azam F. Microbial production of recalcitrant dissolved organic matter: long-term carbon storage in the global ocean. Nature Reviews Microbiology, 2010, 8(8): 593-599. DOI:10.1038/nrmicro2386 |
| [90] | Hopkinson CS, Vallino JJ. Efficient export of carbon to the deep ocean through dissolved organic matter. Nature, 2005, 433(7022): 142-145. DOI:10.1038/nature03191 |
| [91] | Canfield DE, Glazer AN, Falkowski PG. The evolution and future of Earth's nitrogen cycle. Science, 2010, 330(6001): 192-196. DOI:10.1126/science.1186120 |
| [92] | Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Sciences of the United States of America, 2005, 102(41): 14683-14688. DOI:10.1073/pnas.0506625102 |
| [93] | Labrenz M, Sintes E, Toetzke F, Zumsteg A, Herndl GJ, Seidler M, Jürgens K. Relevance of a crenarchaeotal subcluster related to Candidatus Nitrosopumilus maritimus to ammonia oxidation in the suboxic zone of the central Baltic Sea. The ISME Journal, 2010, 4(12): 1496-1508. DOI:10.1038/ismej.2010.78 |
| [94] | Feike J, Jürgens K, Hollibaugh JT, Krüger S, Jost G, Labrenz M. Measuring unbiased metatranscriptomics in suboxic waters of the central Baltic Sea using a new in situ fixation system. The ISME Journal, 2012, 6(2): 461-470. DOI:10.1038/ismej.2011.94 |
| [95] | Berg C, Vandieken V, Thamdrup B, Jürgens K. Significance of archaeal nitrification in hypoxic waters of the Baltic Sea. The ISME Journal, 2015, 9(6): 1319-1332. DOI:10.1038/ismej.2014.218 |
| [96] | Santoro AE, Saito MA, Goepfert TJ, Lamborg CH, Dupont CL, DiTullio GR. Thaumarchaeal ecotype distributions across the equatorial Pacific Ocean and their potential roles in nitrification and sinking flux attenuation. Limnology and Oceanography, 2017, 62(5): 1984-2003. DOI:10.1002/lno.10547 |
| [97] | Zhang Y, Qin W, Hou L, Zakem EJ, Wan X, Zhao Z, Liu L, Hunt KA, Jiao N, Kao S, Tang K, Xie X, Shen J, Li Y, Chen M, Dai X, Liu C, Deng W, Dai M, Ingalls AE, Stahl DA. Nitrifier adaptation to low energy flux controls inventory of reduced nitrogen in the dark ocean. Proceedings of the National Academy of Sciences of the United States of America, 2020, 117(9): 4823-4830. DOI:10.1073/pnas.1912367117 |
| [98] | Ding J, Zhang Y, Wang H, Jian HH, Leng H, Xiao X. Microbial community structure of deep-sea hydrothermal vents on the ultraslow spreading southwest Indian ridge. Frontiers in Microbiology, 2017, 8: 1012. DOI:10.3389/fmicb.2017.01012 |
| [99] | Nakagawa T, Mori K, Kato C, Takahashi R, Tokuyama T. Distribution of cold-adapted ammonia-oxidizing microorganisms in the deep-ocean of the northeastern Japan sea. Microbes and Environments, 2007, 22(4): 365-372. DOI:10.1264/jsme2.22.365 |
| [100] | Sears K, Alleman JE, Barnard JL, Oleszkiewicz JA. Impacts of reduced sulfur components on active and resting ammonia oxidizers. Journal of Industrial Microbiology and Biotechnology, 2004, 31(8): 369-378. DOI:10.1007/s10295-004-0157-2 |
| [101] | Bayer B, Hansman RL, Bittner MJ, Noriega-Ortega BE, Niggemann J, Dittmar T, Herndl GJ. Ammonia-oxidizing archaea release a suite of organic compounds potentially fueling prokaryotic heterotrophy in the ocean. Environmental Microbiology, 2019, 21(11): 4062-4075. DOI:10.1111/1462-2920.14755 |
| [102] | Varela MM, van Aken HM, Sintes E, Reinthaler T, Herndl GJ. Contribution of crenarchaeota and bacteria to autotrophy in the north Atlantic interior. Environmental Microbiology, 2011, 13(6): 1524-1533. DOI:10.1111/j.1462-2920.2011.02457.x |
| [103] | Liu LT, Li SR, Han JM, Lin WT, Luo JF. A two-step strategy for the rapid enrichment of Nitrosocosmicus-like ammonia-oxidizing thaumarchaea. Frontiers in Microbiology, 2019, 10: 875. DOI:10.3389/fmicb.2019.00875 |
| [104] | Kozlowski JA, Stieglmeier M, Schleper C, Klotz MG, Stein LY. Pathways and key intermediates required for obligate aerobic ammonia-dependent chemolithotrophy in bacteria and Thaumarchaeota. The ISME Journal, 2016, 10(8): 1836-1845. DOI:10.1038/ismej.2016.2 |
 2021, Vol. 61
2021, Vol. 61